Healthcare Specialist SHS Capital, together with its co-investors, sells Selfapy GmbH to MEDICE – The Health Family
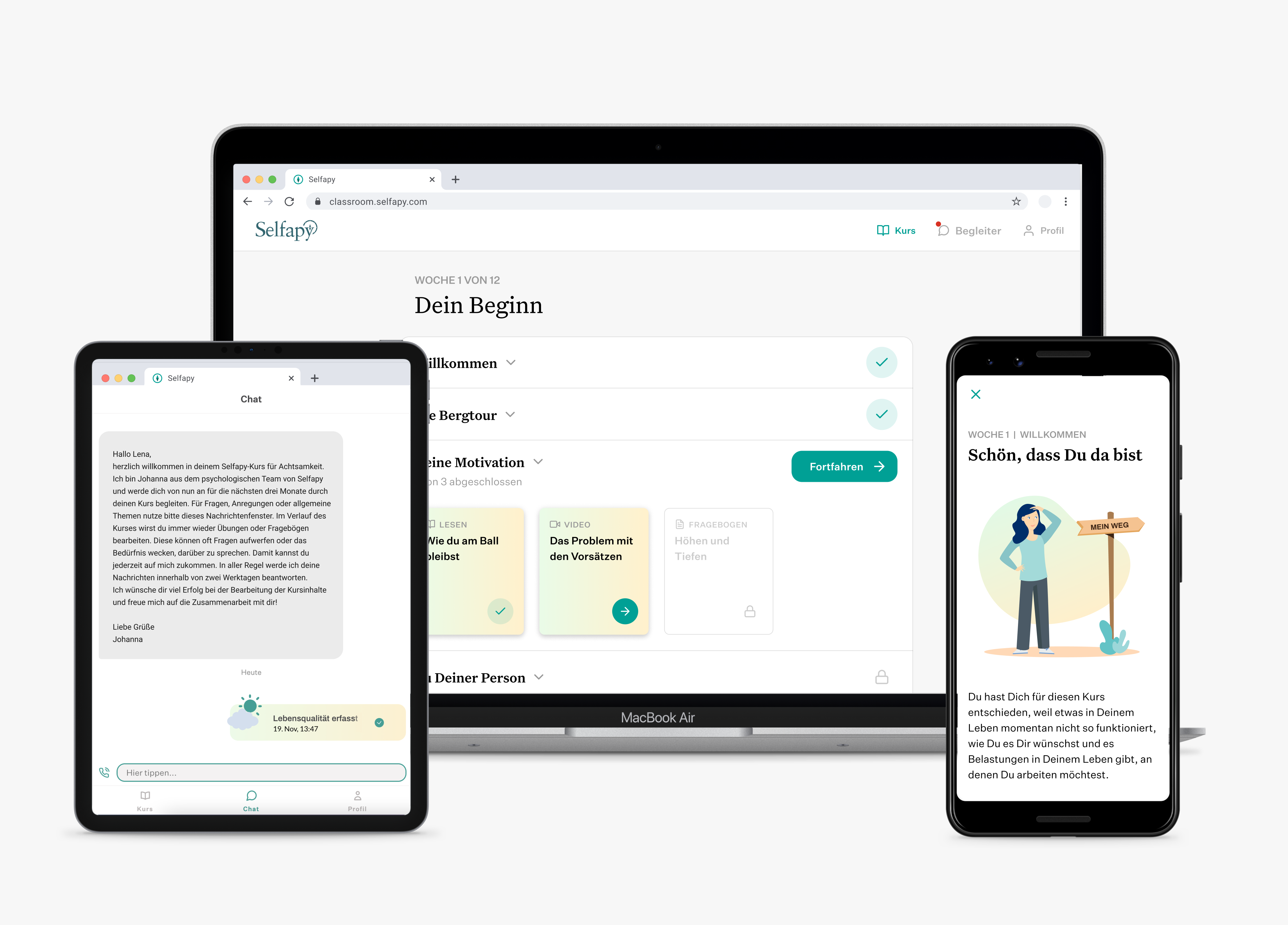
Tuebingen / 13.03.2025 Selfapy GmbH, a digital health pioneer in the field of mental health, has been acquired by MEDICE Health Family, the European market leader in ADHD therapy. As part of the transaction, the investors SHS Capital, Think.Health Ventures, IBB Ventures, and HTGF sold their shares. Selfapy’s CE-certified medical products provide flexible and easily […]









